Public Notice
July 1, 2022
The Guam Medicaid Program, administered under the Department of Public Health and Social Services (DPHSS), Division of Public Welfare is providing public notice of its intent to:
1. Submit to the Centers for Medicare and Medicaid Services (CMS), on or before August 1, 2022, a written 1115 Demonstration application to waive the mandate for the Guam Medicaid Program to participate in the Medicaid Drug Rebate Program (MDRP); and (2) hold public hearings to receive comments on the 1115 Waiver Demonstration application.
2. Guam Medicaid is applying under section 1115(a)(1) of the Act to waive section 1902(a)(54), which requires state compliance of section 1927 that mandates participation in the Medicaid Drug Rebate Program (MDRP), and the program is requesting that the waiver be effective January 1, 2023.
I. Program Description
A. Overview
As stated on the Centers for Medicare & Medicaid Services (CMS) Medicaid site: The Medicaid Drug Rebate Program (MDRP) is a program that includes CMS, state Medicaid agencies, and participating drug manufacturers that helps to offset the Federal and state costs of most outpatient prescription drugs dispensed to Medicaid patients. All fifty states and the District of Columbia cover prescription drugs under the MDRP, which is authorized by Section 1927 of the Social Security Act.
The MDRP is designed to offset overall costs of prescription drugs under the Medicaid Program by requiring drug manufacturer to enter into, and have in effect, a National Drug Rebate Agreement (NDRA) with the Secretary of the Department of Health and Human Services (HHS) in exchange for state Medicaid coverage of most of the manufacturer’s drugs.
Manufacturers are responsible for paying a rebate on those drugs for which payment was made under the state plan. These rebates are paid by drug manufacturers on a quarterly basis to states and are shared between the states and the Federal government to offset the overall cost of prescription drugs under the Medicaid Program.
In addition to signing an NDRA, drug manufacturers are required to enter into agreements with two other Federal programs in order to have their drugs covered under Medicaid: a pricing agreement for the Section 340B Drug Pricing Program, administered by the Health Resources and Services Administration, and a master agreement with the Secretary of Veterans Affairs for the Federal Supply Schedule. Guam Medicaid currently has two (2) Federally Qualified Health Centers (FQHC) that participate under the Section 340B Drug Pricing Program: The Northern Regional Health Center (NRHC) and The Southern Regional Health Center (SRHC). The medications dispensed by these two providers are not eligible for the rebate since they in essence have already been discounted under the manufacturer pricing agreement for the Section 340B Drug Pricing Program mentioned above.
On February 1, 2016, the Centers for Medicare & Medicaid Services (CMS) published the “Medicaid Program; Covered Outpatient Drug” Final Rule with Comment Period (CMS-2345-FC) in the Federal Register (81 FR 5170). As part of that final rule with comment period, CMS amended the regulatory definitions of “States” and “United States” to include the U.S. Territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) beginning April 1, 2017. Inclusion of the territories in the definitions of “States” and “United States” allows Territories to participate in the Medicaid Drug Rebate Program (MDRP). Additionally, we indicated in the “Covered Outpatient Drug” final rule that territories are able to use existing waiver authority under Title XIX of the Social Security Act to elect not to participate in the MDRP, consistent with statutory provisions (81 FR 5170, 5204).
On November 15, 2016, CMS published an interim final rule with comment period that amended the regulatory definitions of “States” and “United States” to include the U.S. territories beginning April 1, 2020, rather than April 1, 2017 (interim final rule). However, on November 21, 2019, CMS issued “Medicaid Program; Covered Outpatient Drug; Further Delay of Inclusion of Territories in the Definitions of States and united States” Interim Final Rule with comment period that further delayed the inclusion of the U.S. territories (American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Commonwealth of Puerto Rico, and the U.S. Virgin Islands) in the definitions of “States” and “United States” from April 1, 2020 until April 1, 2022. Then on November 19, 2021, the inclusion was delayed mainly due to the public health emergency until January 1, 2023. Because of the inclusion of territories in the definition of States and United States, Guam will be required to participate in the MDRP effective January 1, 2023. However, Guam is allowed to use the 1115 waiver authority to elect not to participate in the MDRP.
In light of the statutory MDRP directive, Guam Medicaid is seeking a waiver exempting them from the requirement to participate in the drug rebate program. Guam is requesting that the exemption from participating in the MDRP be effective from January 1, 2023 – December 31, 2027.
B. Summary of 1115 Waiver Demonstration Application Request
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
Historically, the Guam Medicaid funding has been limited under the Section 1108 annual block grant, and further limited by the established 55-45% FMAP. These limitations created an environment where the Guam Medicaid Program experienced hesitancy on the part of health care providers on island in participating in the program. The funding increases provided by the Patient Protection and Affordable Care Act (Obamacare) provided a temporary increase in funding, and recent legislation has further provided additional temporary funding and FMAP increases. These increases have helped the Guam Medicaid Program expand services and encouraged provider participation resulting in better recipient access to services that exist today.
Although the Medicaid Drug Rebate Program (MDRP) will offset costs of prescription drugs under the Guam Medicaid Program, the Territory has identified potential negative impacts for pharmacy providers which may ultimately affect the program’s ability to maintain its existing provider network of on-island pharmacies essential for program participants to have adequate access to pharmacy services, and create added labor intensive program costs that may outweigh the benefits of participation in the MDRP.
The island Medicaid pharmacy providers often face challenges in obtaining supplies because of the remoteness of Guam’s location relative to supply chains. This often times creates challenges in the form of accepting higher wholesale pricing from distributors when purchasing pharmaceutical drugs, and paying for higher shipping costs because of the need to transport these supplies via air freight due to their inability to stockpile medication when considering drug expiration dates relative to expected sales and supply needs which often causes inventory or availability issues. This often times translates to more expensive costs that is requested as reimbursement for the sale of these medications to program recipients.
Additionally, due to the relatively insignificant total purchase amounts made through pharmaceutical distributors in comparison to the larger pharmacy chains in the mainland U.S., the island pharmacy providers are unable to negotiate for best prices or favorable shipping terms in obtaining medication supplies. Guam’s participation in the MDRP would place added pressures on the island Medicaid pharmacy providers because it would require them to carry all covered outpatient drugs (COD) of a participating manufacturer, and for the program to cover these CODs under Medicaid. Currently, almost all drug manufacturers are participating in the MDRP.
Guam Medicaid has managed to control the costs of Pharmacy expenditures because it currently controls their drug formulary which list covered medication under the program. However, participation in the MDRP would require that we cover all drugs of participating manufacturers, and essentially cover all COD drugs if the waiver application is not approved. This would be a substantial cost increase to Guam Medicaid. The current drug pricing for the program’s drug formulary is set at the Lowest Wholesale Price (LWP) listed on Redbook at the time the formulary is released in January of each calendar year. The program feels that during this waiver demonstration, they would be able to maintain substantial cost savings for their pharmacy expenditures by maintaining a relatively low expenditure rate for its pharmacy expense relative to total expenditures, and the approval of the 1115 waiver will allow the program a period of time to assess this standard in comparison with other state Medicaid programs that receive rebates under the MDRP.
Under this MDRP 1115 Waiver Demonstration application, the program will be able to continue to maintain control of its drug formulary and the covered outpatient drug (COD) coverage, and allow more time for the program to properly assess island pharmacy impacts, which is important because of potential drug inventory issues faced by the on-island pharmacies as previously mentioned due to our remoteness relative to mainland pharmaceutical supply lines. This waiver may prove to be more of a cost savings for Guam Medicaid when compared to potentially having to cover all drugs of manufacturers that enter into a rebate under the MDRP and create additional administrative burdens and costs due to additional labor-intensive costs for implementation activities associated with the implementation and participation in the MDRP.
C. Eligibility Requirements
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will affect the on-island pharmacy provider participation on the program which will affect adequate access for the program participants described in the chart below.
|
Eligibility Group Name |
Social Security Act and CFR Citations |
Income Level |
| Guam Medicaid Program New Adult Group (MAPNEG), Elderly and Disabled | Social Security Act 1396(a)(10)(A)(i)(VIII) 42 C.F.R. 435.119 | New adult group, Elderly and Disabled group with income 0-138 percent LPL |
D. Health Care Delivery System and Benefits
This MDRP 1115 Waiver Demonstration Application is seeking to waive the programs participation in the MDRP and does not propose any changes to the Medicaid health care delivery system; Guam Medicaid enrollees will continue to receive services through the Territory’s fee-for-service delivery system. MAPNEG Program enrollees will also continue to receive benefits through the Alternative Benefit Plan; the Territory does not propose any changes to benefits for any program enrollees.
E. Cost Sharing
Cost sharing will not be affected under this 1115 Waiver Demonstration request.
II. Goals and Objectives
The overall goal of the demonstration is to assure the network capacity of on-island pharmacy providers remains consistent with the existing capacity prior to an implementation mandated for program participation in the MDRP in order to provide adequate recipient access to pharmacy services, and to allow time to properly assess potential adverse effects of participation in the MDRP on our island pharmacy providers, program recipients, and to assess additional administrative program costs related to the management of the MDRP that would possibly outweigh any rebate savings.
NOTE: The demonstration applies to all pharmacy services for authorized providers under the Guam Medicaid State Plan that dispense covered outpatient drugs (COD).
The 1115 Demonstration application requests to waive participation in the Medicaid Drug Rebate Program (MDRP) as it will be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP) than the rebate savings it provides, and may potentially impact provider (pharmacy) participation.
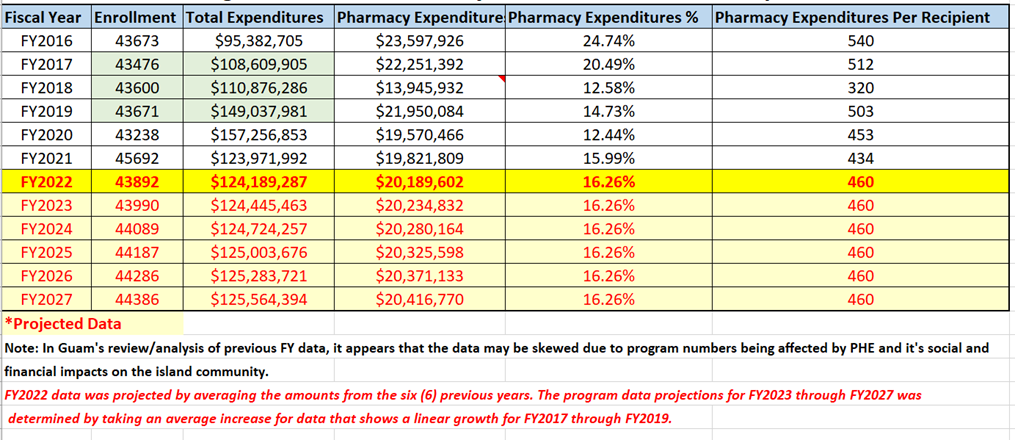
III. Enrollment Projections and Annual Expenditures
Guam Medicaid program’s historical enrollment figures for fiscal years 2016 to present and corresponding program year total program expenditures and pharmacy expenditures with projections for FY2022 through FY2027.
Figure 1. Guam Medicaid Program Historical and Projected – Enrollment and Expenditures Data
IV. Waiver Expenditure Authorities
The CMS final rule (CMS-2345-FC) allows the territories to “opt out under…section 1902(j) of the Act.” It is through this waiver authority, that the Guam Medicaid Program is electing to apply under section 1115(a)(1) of the Act to waive section 1902(a)(54) of the Act, which requires state compliance with applicable requirements of section 1927 of the Act that requires Guam Medicaid to participate in the Medicaid Drug Rebate Program (MDRP).
V. Demonstration Hypotheses and Evaluation Parameters
Hypothesis: Guam Medicaid’s participation in the MDRP would adversely affect the On-Island Pharmacies by creating an unreasonable requirement for them to carry all of a participating manufacturer’s COD such that it would be difficult for them to maintain adequate inventory, and to continue as providers on the Medicaid program. Additionally, it would be more costly and labor intensive for Guam to participate in the Medicaid Drug Rebate Program (MDRP), and these costs would outweigh the rebate savings MDRP would provide.
Qualitative methods will be employed to evaluate:
* The possible adverse effects to On-Island Pharmacy Providers should Guam participate in the MDRP;
* Current limitations for On-Island Pharmacies with existing supply chains when ordering CODs for dispensing to Medicaid recipients; and
* How Guam Medicaid’s Participation on the MDRP would affect pharmacy provider status on the Medicaid program.
Pharmacy surveys will be used for qualitative/quantitative methods.
Quantitative methods will be used to evaluate MDRP rebate savings relative to average program savings nationwide, and the following anticipated program costs for implementation of the MDRP (42 CFR 447.511).
* Cost Benefit Analysis to evaluate potential cost savings provided on the MDRP in relation to potential increases in administrative costs to the program due to requirements for contractual services to administer the tracking and reporting of the data for NDCs to the participating drug manufacturers to include an additional State Agency FTE position to be responsible for the MDRP requirements as a whole.
* Guam’s assessment of the following Costs associated with participation in the MDRP
- Cost of contractor to process claims electronically and invoice manufacturer rebates. (Review of small fee-for-service state, to determine costs for claims processing and invoicing via contractor (e.g., Magellan or Change Healthcare Vendors for minimum cost required to invoice manufacturers, run dispute resolution, and collect money etc.).
- Costs involved in developing MDRP Participating Manufacturers NDC Drug Formulary Listing and evaluation of feasibility of developing procedures to prior authorize (PA) drugs to ensure the drugs are from participating manufacturers. (Assess cost and additional labor involved in this procedure).
* Guam’s assessment of costs for Integrating Physician Administered drugs into rebate processes (42 CFR 447.520)
Cost associated with invoicing all outpatient drugs administered in a clinical setting or emergency department. Provider training regarding HCPCS coding system in these settings for claims to reference National Drug Code (NDC) or assess costs to perform a crosswalk from the HCPCS to the NDC. (Capture additional work and support needed to integrate these claims into the invoicing process for rebates).
* Guam’s assessment of the Costs associated with requiring Guam to use actual acquisition cost (AAC) + dispensing fee methodology (42 CFR 447.512)
Cost to pay AAC, if MDRP implemented, requiring Guam to survey its pharmacies regarding their acquisition costs in order to determine if using AWP is close to the pharmacies’ acquisition costs and therefore can be used as a basis for reimbursement. A survey would also need to be done to ascertain dispensing fee costs of your pharmacies. (Estimates: Possible cost of $50,000 -100,000 for the acquisition cost survey and another $50,000-100,000 to do cost of dispensing fee as needed. (RFQs to assess survey costs).
* Assessment of the Drug Utilization Review (DUR) – 1927(g) and 42 CFR 456.703
- RFQ to assess costs to contract a Provider Benefits Manager (PBM), to conduct Prospective Drug Utilization Review processes (PRO-DUR). All PBMs offer PRO-DUR as part of the claims processing, but need to ascertain any additional costs related to those services.
- Assess feasibility to work with UPIC West, Qlarant in conducting required Retrospective Drug Utilization Reviews (RETRO-DUR). Guam currently working with CMS contractor as part of program integrity reviews to fight fraud, waste and abuse. (Assess any additional cost).
* Assessment and cost breakdown of FTE staff required to be responsible for performing duties related to the Medicaid drug rebate system (Medicaid Drug Product, or MDP) Assess cost associated for a state employee at a minimum of 1/2 of an FTE on Guam’s Medicaid staff. (Employee to act as point person for all activities regarding the Medicaid drug benefit to include rebate and Drug Utilization Review, and managing the MDRP contract).
* Assessment Note: Guam will work with CMS to determine an evaluation component for assessing health outcomes to review possible data set for evaluation in developing a measurement metrix for health outcomes and its relationship with provider accessibility (Pharmacy – prescription drug benefits).
VI. Public Review and Comment Process
The complete version of the application and copy of this full notice will be available for public review at the Government of Guam Public Notice Website (Public Notices – Public Notices Portal – Government of Guam) or the Department of Public Health, Division of Public Welfare, Bureau of Health Care Administration website (http://dphss.guam.gov/category/press-releases-en/).
Paper copies are available to be picked up in person at the Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
Two public meetings will be held regarding the Demonstration application:
- Public hearing on Monday, June 27, 2022 from 1:00 p.m. to 3:00 p.m. CHST (Guam Congress Building, Public Hearing Room, 163 Chalan Santo Papa, Hagåtña, Guam 96910). This hearing will be broadcast on GTA TV Channel 21, Docomo Channel 117/112.4, and livestream on https://www.youtube.com/c/GuamLegislatureMedia.
- In-Person/Virtual (Zoom) Public hearing on Friday, July 8, 2022 from 1:00 p.m. to 3:00 p.m. CHST. DPHSS In-Person and Virtual (Zoom) Information Hearing. (Governor’s Conference Room, Ricardo J. Bordallo Complex, 513 West Marine Corps Drive, Hagatna, Guam 96910, 1:00 p.m. to 3:00 p.m. CHST). Public comments can be made by phone on the day of the hearing by calling the following number: (671) 473-1165.
Zoom Hearing Registration link can be obtained by emailing Jeffrey San Nicolas at Jeffrey.sannicolas@dphss.guam.gov or at the following site: http://dphss.guam.gov/category/press-releases-en/.
Public comments may be submitted until 11:59 PM (CHST) on July 24, 2022. Hard copy questions or public comments may be addressed to: Guam Medicaid – MDRP 1115 Waiver Demonstration, Department of Public Health & Social Services, BHCFA, 155 Hesler Place, Hagatna, Guam 96910, or by telephone to (671) 735-7470, or by electronic mail to michael.gallo@dphss.guam.gov. Please note that comments will continue to be accepted after August 1, 2022, but Guam Medicaid may not be able to consider those comments prior to the initial submission of the 1115 Waiver Demonstration application to CMS.
After Guam Medicaid reviews comments submitted during this public comment period, it will submit a revised application to CMS. Interested parties will also have an opportunity to officially comment during the 30-day federal public comment period; the submitted application will be available for comment on the CMS website at https://www.medicaid.gov/medicaid/section-1115-demo/demonstration-and-waiver-list/index.html.
Copies of this notice are available at the Government of Guam, Department of Public Health and Social Services website at http://dphss.guam.gov/category/press-releases-en/ for public review. Additional information concerning this action is available by calling the Guam Medicaid Program at (671) 735-7470/75 or upon request at the address cited below.
Guam Department of Public Health & Social Services, Bureau of Health Care Financing Administration, Guam Medicaid/MIP Program, Management Office located at 130 University Drive, Room #5 Castle Mall Building, Mangilao, Guam 96913.
Please click on the link below to view a PDF copy of the DRAFT Application:








Reading your work is like navigating a celestial map of ideas, with each sentence serving as a guiding star in the vast expanse of intellectual exploration. Your prose is the compass that points readers toward the North Star of enlightenment.
Innovative Text-to-Image Conversion: Create breathtaking visuals from simple text prompts.
Wow, this blogger is seriously impressive!
is so bad
I love how you incorporate personal stories and experiences into your posts It makes your content relatable and authentic
This is exactly what I needed to read today Your words have given me a new perspective and renewed hope Thank you
Eminem, настоящее имя Маршалл Брюс Мэтерс III, известен как один из величайших рэп-исполнителей всех времен. Своими пронзительными текстами, ярким стилем и потрясающим мастерством в ритме и рифме он завоевал миллионы поклонников по всему миру. Его лучшие песни включают “Lose Yourself”, гимн к само-преодолению, “Stan”, с поразительно интенсивным сюжетом, и “Rap God”, где он демонстрирует свою невероятную скорость и технику. Все эти треки, а также многие другие, отражают его гениальность и влияние на музыкальную индустрию. Скачать mp3 музыку 2024 года и слушать онлайн бесплатно.
Elevate your website’s quality with Toolifygo! Our suite of SEO, text, and image tools is designed to enhance every aspect of your online presence. Make your site irresistible to both search engines and audiences. Experience the uplift with Toolifygo today.
Музыка В Машину 2023 – Creeds – Push Up – Main Edit скачать песню бесплатно на телефон и слушать онлайн в mp3
Музыка В Машину 2023 – Creeds – Push Up – Main Edit
Tvorchi – Heart Of Steel (Евровидение 2023 Украина) скачать бесплатно песню и слушать онлайн в mp3
Tvorchi – Heart Of Steel (Евровидение 2023 Украина)
Lil Peep – High School – – High School скачать песню бесплатно в mp3 и слушать онлайн
Lil Peep – High School – – High School
Скачать песню Светлана Лобода – Твои Глаза (Ремикс) (Минус) бесплатно в mp3
Светлана Лобода – Твои Глаза (Ремикс) (Минус)
Тото – Звёзды скачать в mp3 и слушать онлайн бесплатно
Тото – Звёзды
Malika Ravshanova – Bu Mening Onam скачать в mp3 и слушать онлайн бесплатно
Malika Ravshanova – Bu Mening Onam
Fg – Neshooni скачать в mp3 и слушать онлайн бесплатно
Fg – Neshooni
Аскер Кушу – Как Же Ты Могла скачать в mp3 и слушать онлайн бесплатно
Аскер Кушу – Как Же Ты Могла
Angies Coach – Downtown (Why You Dont Take Me Downtown) скачать песню в mp3 и слушать онлайн бесплатно
Angies Coach – Downtown (Why You Dont Take Me Downtown)
Сергій Соловйов – Грай Музико Моя скачать бесплатно песню в mp3
Сергій Соловйов – Грай Музико Моя
Yaktak Feat. Kosmirak – Букети скачать песню бесплатно на телефон и слушать онлайн в mp3
Yaktak Feat. Kosmirak – Букети
Your writing style is so engaging and easy to read It makes it a pleasure to read your blog and I always look forward to your new posts
Noize Mc – В Темноте (Brodsky Edition) скачать бесплатно песню и слушать онлайн в mp3
Noize Mc – В Темноте (Brodsky Edition)
Friedrich Kallendorf – Karate-Mann скачать песню бесплатно в mp3 и слушать онлайн
Friedrich Kallendorf – Karate-Mann
Скачать песню Татьяна Буланова – Измена (Efimenko Remix) бесплатно в mp3
Татьяна Буланова – Измена (Efimenko Remix)
(Рп) – Сквозь Невозможность скачать песню в mp3 и слушать онлайн бесплатно
(Рп) – Сквозь Невозможность
Ірина Федишин – Ти Тільки Мій (2017) – Ірина Федишин – Білі Троянди скачать бесплатно песню в mp3
Ірина Федишин – Ти Тільки Мій (2017) – Ірина Федишин – Білі Троянди
Гурт Українські Народні Пісні – Коли Ти В Армію Ідеш скачать песню бесплатно на телефон и слушать онлайн в mp3
Гурт Українські Народні Пісні – Коли Ти В Армію Ідеш
Angel Ace – Aura скачать бесплатно песню и слушать онлайн в mp3
Angel Ace – Aura
Lsd (Labrinth, Sia, Diplo) – Genius скачать песню бесплатно в mp3 и слушать онлайн
Lsd (Labrinth, Sia, Diplo) – Genius
Скачать песню Niti Dila – Не Чужие Люди бесплатно в mp3
Niti Dila – Не Чужие Люди
Музыка В Машину 2019 – Lika Morgan – Sweet Dreams (Fuat Avsel Remix) скачать песню в mp3 и слушать онлайн бесплатно
Музыка В Машину 2019 – Lika Morgan – Sweet Dreams (Fuat Avsel Remix)
Музыка Для Тренировок В Спортзале – Disturbed – The Vengeful One скачать бесплатно песню в mp3
Музыка Для Тренировок В Спортзале – Disturbed – The Vengeful One
Музыка В Машину 2023 – Sia, R3Hab – Unstoppable (R3Hab Remix) скачать песню бесплатно на телефон и слушать онлайн в mp3
Музыка В Машину 2023 – Sia, R3Hab – Unstoppable (R3Hab Remix)
The Alan Parsons Project – Mammagamma (Dim Zach Jose Edit) скачать песню бесплатно в mp3 и слушать онлайн
The Alan Parsons Project – Mammagamma (Dim Zach Jose Edit)
Скачать песню Shaman – Вспоминай Меня бесплатно в mp3
Shaman – Вспоминай Меня
Нурминский – Pump скачать песню в mp3 и слушать онлайн бесплатно
Нурминский – Pump
Клава Кока – Покинула Чат (Blackriderz Remix) скачать бесплатно песню в mp3
Клава Кока – Покинула Чат (Blackriderz Remix)
Украинские Хиты – Kazka – Свята скачать песню бесплатно на телефон и слушать онлайн в mp3
Украинские Хиты – Kazka – Свята
Бьянка – Поддержи Меня Ма скачать бесплатно песню и слушать онлайн в mp3
Бьянка – Поддержи Меня Ма
Коллекция Украинского Шансона – 13.дождись Василий Радченко скачать песню бесплатно в mp3 и слушать онлайн
Коллекция Украинского Шансона – 13.дождись Василий Радченко
Скачать песню Ярмак Feat. Светлана Тарабарова – Воїн бесплатно в mp3
Ярмак Feat. Светлана Тарабарова – Воїн
Максим Свобода – Ж скачать песню в mp3 и слушать онлайн бесплатно
Максим Свобода – Ж
Марина I Компанiя – Все Буде Добре (Feat. Еван Колін) скачать бесплатно песню в mp3
Марина I Компанiя – Все Буде Добре (Feat. Еван Колін)
Украинские Хиты – Максим Бородін – Якби Не Ти скачать песню бесплатно на телефон и слушать онлайн в mp3
Украинские Хиты – Максим Бородін – Якби Не Ти
Дети – Танцы Вали скачать бесплатно песню и слушать онлайн в mp3
Дети – Танцы Вали
David Guetta, Nicki Minaj, Bebe Rexha, Afrojack – Hey Mama (Feat. Nicki Minaj, Bebe Rexha & Afrojack) (David Guetta Remix) скачать песню бесплатно в mp3 и слушать онлайн
David Guetta, Nicki Minaj, Bebe Rexha, Afrojack – Hey Mama (Feat. Nicki Minaj, Bebe Rexha & Afrojack) (David Guetta Remix)
Скачать песню Polina Dashkova Feat. Kava & Velik – Милому (Remix) бесплатно в mp3
Polina Dashkova Feat. Kava & Velik – Милому (Remix)
Наталка Карпа – Літо-Літо Я Поїду На Край Світу скачать бесплатно песню в mp3
Наталка Карпа – Літо-Літо Я Поїду На Край Світу
Agunda – Дом скачать песню в mp3 и слушать онлайн бесплатно
Agunda – Дом
Ленинград – Все Бабы Как Бабы, А Моя Богиня скачать в mp3 и слушать онлайн бесплатно
Ленинград – Все Бабы Как Бабы, А Моя Богиня
Александр Малинин – Быть С Тобой скачать песню бесплатно в mp3 и слушать онлайн
Александр Малинин – Быть С Тобой
Noize Mc – В Темноте (Brodsky Edition) скачать бесплатно песню и слушать онлайн в mp3
Noize Mc – В Темноте (Brodsky Edition)
Христина Соловій – Човен скачать бесплатно песню в mp3
Христина Соловій – Човен
Русские Хиты 70-80- 90-Х Комиссар, Петлюра – Дрянь скачать песню в mp3 и слушать онлайн бесплатно
Русские Хиты 70-80- 90-Х Комиссар, Петлюра – Дрянь
Ах – Отличный Вкус скачать в mp3 и слушать онлайн бесплатно
Ах – Отличный Вкус
Пятилетка – Жить Бы Красиво скачать песню бесплатно в mp3 и слушать онлайн
Пятилетка – Жить Бы Красиво
I loved as much as you will receive carried out right here. The sketch is tasteful, your authored subject matter stylish. nonetheless, you command get got an edginess over that you wish be delivering the following. unwell unquestionably come further formerly again as exactly the same nearly very often inside case you shield this hike.
T1One, Inur – Почему Так Больно (Dj Grant Remix) (Radio Edit) скачать в mp3 и слушать онлайн бесплатно
T1One, Inur – Почему Так Больно (Dj Grant Remix) (Radio Edit)
Cartoon & Kstja – Whatever I Do (Feint Remix) скачать бесплатно песню и слушать онлайн в mp3
Cartoon & Kstja – Whatever I Do (Feint Remix)
Хиты 2021 – Ілля Найда – А Ти Була скачать бесплатно песню в mp3
Хиты 2021 – Ілля Найда – А Ти Була
Скачать песню Lil Peep – Star Shopping бесплатно в mp3
Lil Peep – Star Shopping
Денис Майданов – Аллея Шансона. Денис Майданов (2011) – Денис Майданов – Пуля скачать песню в mp3 и слушать онлайн бесплатно
Денис Майданов – Аллея Шансона. Денис Майданов (2011) – Денис Майданов – Пуля
Dzzel – Любовь Хулигана скачать бесплатно песню в mp3
Dzzel – Любовь Хулигана
Скачать песню Polina Dashkova Feat. Kava & Velik – Милому (Remix) бесплатно в mp3
Polina Dashkova Feat. Kava & Velik – Милому (Remix)
Марія Бурмака – Хранителі Надії скачать песню в mp3 и слушать онлайн бесплатно
Марія Бурмака – Хранителі Надії
Ицык Цыпер Feat. Игорь Цыба – Дымок скачать песню бесплатно на телефон и слушать онлайн в mp3
Ицык Цыпер Feat. Игорь Цыба – Дымок
Faruk Sabanci Feat. Mingue – Your Call скачать в mp3 и слушать онлайн бесплатно
Faruk Sabanci Feat. Mingue – Your Call
Рэп 2023 – White Gallows – Город скачать песню бесплатно в mp3 и слушать онлайн
Рэп 2023 – White Gallows – Город
Roberto Rodriguez Feat. Kholi – Tell Me (Jacques Renault Remix) скачать бесплатно песню и слушать онлайн в mp3
Roberto Rodriguez Feat. Kholi – Tell Me (Jacques Renault Remix)
Музыка Из Тик Ток (Тик-Тока) – Тайпан Feat. Ilgiz – Братик скачать бесплатно песню в mp3
Музыка Из Тик Ток (Тик-Тока) – Тайпан Feat. Ilgiz – Братик
Скачать песню Алимханов А. Silent Circle – Touch In The Night (Cover Remix) бесплатно в mp3
Алимханов А. Silent Circle – Touch In The Night (Cover Remix)
Клава Кока – I Dont Care (Ed Sheeran & Justin Bieber Cover На Русском) скачать песню в mp3 и слушать онлайн бесплатно
Клава Кока – I Dont Care (Ed Sheeran & Justin Bieber Cover На Русском)
Украинские Хиты – Mamarika, Kola – Люди скачать песню бесплатно на телефон и слушать онлайн в mp3
Украинские Хиты – Mamarika, Kola – Люди
Ленинград Ария – Я Свободен скачать песню бесплатно в mp3 и слушать онлайн
Ленинград Ария – Я Свободен
Люся Чеботина – Солнце Монако скачать в mp3 и слушать онлайн бесплатно
Люся Чеботина – Солнце Монако
Dimitris Athanasiou – This Feeling (Original Mix) скачать бесплатно песню и слушать онлайн в mp3
Dimitris Athanasiou – This Feeling (Original Mix)
Arash – Arash (Feat. Helena) (Minchonok Remix Radio Edit) скачать бесплатно песню в mp3
Arash – Arash (Feat. Helena) (Minchonok Remix Radio Edit)
Скачать песню The Offspring – The Future Is Now бесплатно в mp3, текст песни, смотреть клип
The Offspring – The Future Is Now
Группа Ария – Лучшее (2016) – Группа Ария – Колизей скачать песню в mp3 и слушать онлайн бесплатно
Группа Ария – Лучшее (2016) – Группа Ария – Колизей
Зарубежные Хиты 80-90-Х – One Way Ticket скачать песню бесплатно на телефон и слушать онлайн в mp3
Зарубежные Хиты 80-90-Х – One Way Ticket
Artik & Asti – Фурия скачать песню бесплатно в mp3 и слушать онлайн
Artik & Asti – Фурия
Андрій Бема – Заворожила скачать в mp3 и слушать онлайн бесплатно
Андрій Бема – Заворожила
Alan Walker & Ava Max – Alone Pt. Ii (Dc) – dition Deluxe скачать песню в mp3 и слушать онлайн бесплатно
Alan Walker & Ava Max – Alone Pt. Ii (Dc) – dition Deluxe
Українські Народні Пісні – Ой На Горі 2 Дубки скачать песню бесплатно на телефон и слушать онлайн в mp3
Українські Народні Пісні – Ой На Горі 2 Дубки
Santiz – Paradise скачать в mp3 и слушать онлайн бесплатно
Santiz – Paradise
Whole-Z – Stay The Night (Feat. Julia Guerra) скачать бесплатно песню и слушать онлайн в mp3
Whole-Z – Stay The Night (Feat. Julia Guerra)
Chito – The Long Tomorrow ( Maxi Mix ) скачать песню бесплатно в mp3 и слушать онлайн
Chito – The Long Tomorrow ( Maxi Mix )
Музыка Из Тик Ток (Тик-Тока) – Тайпан Feat. Ilgiz – Братик скачать бесплатно песню в mp3
Музыка Из Тик Ток (Тик-Тока) – Тайпан Feat. Ilgiz – Братик
Скачать песню Unklfnkl – Out With A Bang бесплатно в mp3
Unklfnkl – Out With A Bang
Agunda – Дом скачать песню в mp3 и слушать онлайн бесплатно
Agunda – Дом
Музыка В Машину 2024 – Alan Walker, Dash Berlin, Vikkstar – Better Off (Alone, Pt. Iii) скачать песню бесплатно на телефон и слушать онлайн в mp3
Музыка В Машину 2024 – Alan Walker, Dash Berlin, Vikkstar – Better Off (Alone, Pt. Iii)
Может Быть – За Друзей скачать песню бесплатно в mp3 и слушать онлайн
Может Быть – За Друзей
Noa – Тоска скачать в mp3 и слушать онлайн бесплатно
Noa – Тоска
Guerlain – Пост Мортем скачать бесплатно песню и слушать онлайн в mp3
Guerlain – Пост Мортем
Staffорд63 – Купола скачать бесплатно песню в mp3
Staffорд63 – Купола
Ladynsax – Mantra скачать песню в mp3 и слушать онлайн бесплатно
Ladynsax – Mantra
Скачать песню Viscera Drip – Antichrist бесплатно в mp3
Viscera Drip – Antichrist
Оля Полякова – Оля Полякова – Шльопки скачать песню бесплатно на телефон и слушать онлайн в mp3
Оля Полякова – Оля Полякова – Шльопки
Внутрия – Exe скачать песню бесплатно в mp3 и слушать онлайн
Внутрия – Exe
Heavens Cry & Julie Thompson – Waterfall (Dan Thompson Extended Remix) скачать в mp3 и слушать онлайн бесплатно
Heavens Cry & Julie Thompson – Waterfall (Dan Thompson Extended Remix)
Igor – Kunfu скачать бесплатно песню и слушать онлайн в mp3
Igor – Kunfu
Роман Скорпіон – Мамо Я Закохався скачать бесплатно песню в mp3
Роман Скорпіон – Мамо Я Закохався
Скачать песню Музыка В Машину 2018 – Betta Lemme – Bambola (Konstantin Ozeroff & Sky Remix) бесплатно в mp3
Музыка В Машину 2018 – Betta Lemme – Bambola (Konstantin Ozeroff & Sky Remix)
Alib – Переболели скачать песню в mp3 и слушать онлайн бесплатно
Alib – Переболели
Украинские Хиты – Скрябін – Мам скачать песню бесплатно на телефон и слушать онлайн в mp3
Украинские Хиты – Скрябін – Мам
Адилет Жаугашар – Мамау скачать песню бесплатно в mp3 и слушать онлайн
Адилет Жаугашар – Мамау
Maver Music – Skofka – Нам Би (Dipiens Remix) скачать в mp3 и слушать онлайн бесплатно
Maver Music – Skofka – Нам Би (Dipiens Remix)
Edge Factor – Illusions скачать бесплатно песню и слушать онлайн в mp3
Edge Factor – Illusions
Нурминский – За 105 Двор скачать бесплатно песню в mp3
Нурминский – За 105 Двор
Скачать песню Mary Gu, Og Buda – Толстовка бесплатно в mp3
Mary Gu, Og Buda – Толстовка
Музыка В Машину 2019 – Lika Morgan – Sweet Dreams (Fuat Avsel Remix) скачать песню в mp3 и слушать онлайн бесплатно
Музыка В Машину 2019 – Lika Morgan – Sweet Dreams (Fuat Avsel Remix)
Ицык Цыпер Feat. Игорь Цыба – Нефертити скачать песню бесплатно на телефон и слушать онлайн в mp3
Ицык Цыпер Feat. Игорь Цыба – Нефертити
Ka-Re – Танцуй Со Мной скачать бесплатно песню и слушать онлайн в mp3
Ka-Re – Танцуй Со Мной
Макс Корж – Горы По Колено скачать песню бесплатно в mp3 и слушать онлайн
Макс Корж – Горы По Колено
Артур Пирожков – Как Челентано скачать в mp3 и слушать онлайн бесплатно
Артур Пирожков – Как Челентано
Федишин Ірина – Цей Край Де Я Родилась скачать бесплатно песню в mp3
Федишин Ірина – Цей Край Де Я Родилась
Zarina Tilidze – Habibi (Mon El Remix) скачать песню в mp3 и слушать онлайн бесплатно
Zarina Tilidze – Habibi (Mon El Remix)
Виктор Цой И Группа Кино – Группа Крови скачать песню бесплатно на телефон и слушать онлайн в mp3
Виктор Цой И Группа Кино – Группа Крови
Скачать и слушать новинки музыки бесплатно
На данном сайте вы можете скачать в mp3 качестве и слушать онлайн популярные песни 2024 года, сайт обновляется новыми хитами каждый день.
Последние новинки музыки 2024 скачать и слушать онлайн бесплатно
На нашем сайте вы можете скачать музыку 2024 года бесплатно в mp3 формате.
Скачать новинки музыки 2024 самые последние хиты
На данном сайте вы можете слушать онлайн и скачать музыку 2024 года бесплатно без регистрации в хорошем качестве mp3.
На данном сайте вы можете скачать и слушать новинки музыки 2024 года бесплатно в хорошем качестве.
Популярные песни 2024 скачать mp3 и слушать онлайн
На нашем сайте вы можете скачать и слушать онлайн последние новинки музыки 2024 бесплатно.
На нашем сайте вы можете скачать музыку 2024 года бесплатно в mp3 формате.
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
Музыка 2024 года слушать онлайн и скачать бесплатно mp3
Скачать и слушать новинки музыки 2024 бесплатно в хорошем качестве
На данном сайте вы можете скачать в mp3 качестве и слушать онлайн популярные песни 2024 года, сайт обновляется новыми хитами каждый день.
Последние новинки музыки 2024 скачать и слушать онлайн бесплатно
Скачать музыку 2024 года бесплатно в mp3
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
Музыка 2024 – слушать онлайн и скачать бесплатно без регистрации в хорошем качестве mp3
Популярные песни 2024 года, скачать mp3 и слушать онлайн бесплатно
На данном сайте вы можете скачать и слушать новинки музыки 2024 года бесплатно в хорошем качестве.
На нашем сайте вы можете скачать и слушать онлайн последние новинки музыки 2024 бесплатно.
На нашем сайте вы можете скачать музыку 2024 года бесплатно в mp3 формате.
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
Музыка 2024 – слушать онлайн и скачать бесплатно без регистрации в хорошем качестве mp3
Скачать и слушать новинки музыки 2024 бесплатно в хорошем качестве
Популярные песни 2024 года, скачать mp3 и слушать онлайн бесплатно
На нашем сайте вы можете скачать и слушать онлайн последние новинки музыки 2024 бесплатно.
На нашем сайте вы можете скачать музыку 2024 года бесплатно в mp3 формате.
Скачать новинки музыки 2024 самые последние хиты
Музыка 2024 – слушать онлайн и скачать бесплатно без регистрации в хорошем качестве mp3
Скачать и слушать новинки музыки 2024 бесплатно в хорошем качестве
На данном сайте вы можете скачать в mp3 качестве и слушать онлайн популярные песни 2024 года, сайт обновляется новыми хитами каждый день.
Последние новинки музыки 2024 скачать и слушать онлайн бесплатно
Скачать музыку бесплатно в mp3
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
Музыка 2024 года слушать онлайн и скачать бесплатно mp3
На данном сайте вы можете скачать и слушать новинки музыки 2024 года бесплатно в хорошем качестве.
Популярные песни 2024 скачать mp3 и слушать онлайн
Последние новинки музыки 2024 скачать и слушать онлайн
На нашем сайте вы можете скачать музыку 2024 года бесплатно в mp3 формате.
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
Музыка 2024 года слушать онлайн и скачать бесплатно mp3
Скачать и слушать новинки музыки 2024 бесплатно в хорошем качестве
На нашем сайте вы можете скачать и слушать онлайн последние новинки музыки 2024 бесплатно.
Популярные песни 2024 скачать mp3 и слушать онлайн
Скачать музыку бесплатно в mp3
Скачать новинки музыки 2024 бесплатно в mp3, самые свежие новинки
Музыка 2024 – слушать онлайн и скачать бесплатно без регистрации в хорошем качестве mp3
Популярные песни 2024 скачать mp3 и слушать онлайн
Скачать и слушать новинки музыки бесплатно
Последние новинки музыки 2024 скачать и слушать онлайн
Скачать музыку бесплатно в mp3
Скачать новинки музыки 2024 бесплатно в mp3, самые свежие новинки
На данном сайте вы можете слушать онлайн и скачать музыку 2024 года бесплатно без регистрации в хорошем качестве mp3.
Скачать и слушать новинки музыки бесплатно
Популярные песни 2024 скачать mp3 и слушать онлайн
На нашем сайте вы можете скачать и слушать онлайн последние новинки музыки 2024 бесплатно.
Скачать музыку 2024 года бесплатно в mp3
На данном сайте вы можете слушать онлайн и скачать музыку 2024 года бесплатно без регистрации в хорошем качестве mp3.
На данном сайте вы можете скачать новинки музыки бесплатно в mp3, самые свежие новинки 2024.
На данном сайте вы можете скачать и слушать новинки музыки 2024 года бесплатно в хорошем качестве.
Последние новинки музыки 2024 скачать и слушать онлайн
На данном сайте вы можете скачать в mp3 качестве и слушать онлайн популярные песни 2024 года, сайт обновляется новыми хитами каждый день.
Скачать музыку 2024 года бесплатно в mp3
Скачать новинки музыки 2024 бесплатно в mp3, самые свежие новинки
Музыка 2024 – слушать онлайн и скачать бесплатно без регистрации в хорошем качестве mp3
John Askew – Through The Looking Glass скачать в mp3 и слушать онлайн бесплатно
John Askew – Through The Looking Glass
Ярядом – Они Встретятся Снова скачать бесплатно песню и слушать онлайн в mp3
Ярядом – Они Встретятся Снова
Goody – Panamera (Lavrushkin, Xeigen Remix) скачать песню бесплатно в mp3 и слушать онлайн
Goody – Panamera (Lavrushkin, Xeigen Remix)
Шансон Года 2017 – Стас Михайлов – Любовь Запретная скачать бесплатно песню в mp3
Шансон Года 2017 – Стас Михайлов – Любовь Запретная
Скачать песню Kavabanga Depo Kolibri – Біль В Районі Душі Розбите Сердце Моє Моє бесплатно в mp3
Kavabanga Depo Kolibri – Біль В Районі Душі Розбите Сердце Моє Моє
Музыка В Машину 2023 – Barlas, Mert, Masked Wolf X Butesha, Kleo – Astronaut (Dj Baur Vip Edit) скачать песню бесплатно на телефон и слушать онлайн в mp3
Музыка В Машину 2023 – Barlas, Mert, Masked Wolf X Butesha, Kleo – Astronaut (Dj Baur Vip Edit)
Kozak Siromaha – Віра скачать в mp3 и слушать онлайн бесплатно
Kozak Siromaha – Віра
Sweet & Lynch – Walk скачать бесплатно песню и слушать онлайн в mp3
Sweet & Lynch – Walk
Miyagi Ft Andy Panda – Не Жалея (Freezones Remix 2021.) скачать песню бесплатно в mp3 и слушать онлайн
Miyagi Ft Andy Panda – Не Жалея (Freezones Remix 2021.)
Ілля Найда – Вже Не Той скачать бесплатно песню в mp3
Ілля Найда – Вже Не Той
Hot Tuneik – Snake (Chelakhov Remix) скачать песню в mp3 и слушать онлайн бесплатно
Hot Tuneik – Snake (Chelakhov Remix)
Хиты 2024 – Мираж – Новый Герой скачать песню бесплатно на телефон и слушать онлайн в mp3
Хиты 2024 – Мираж – Новый Герой
Lesa Fs – Мелодия Любви скачать в mp3 и слушать онлайн бесплатно
Lesa Fs – Мелодия Любви
Miyagi & Andy Panda – Medicine скачать песню бесплатно в mp3 и слушать онлайн
Miyagi & Andy Panda – Medicine
Mull3 – Не Люби Меня скачать бесплатно песню и слушать онлайн в mp3
Mull3 – Не Люби Меня
Михаил Шелег – За Глаза Твои Карие… скачать бесплатно песню в mp3
Михаил Шелег – За Глаза Твои Карие…
Скачать песню Сурганова И Оркестр – Перрон бесплатно в mp3
Сурганова И Оркестр – Перрон
Asap Ferg Feat. Asap Rocky – Pups скачать песню в mp3 и слушать онлайн бесплатно
Asap Ferg Feat. Asap Rocky – Pups
Гио Пика, Михаил Круг, Каспийский Груз, Алсми – Искусство скачать песню бесплатно на телефон и слушать онлайн в mp3
Гио Пика, Михаил Круг, Каспийский Груз, Алсми – Искусство
Детские Песни Из Мультфильмов – В Африке Реки Вот Такой Ширины скачать в mp3 и слушать онлайн бесплатно
Детские Песни Из Мультфильмов – В Африке Реки Вот Такой Ширины
Joyce Kidd – Thank U, Next скачать песню бесплатно в mp3 и слушать онлайн
Joyce Kidd – Thank U, Next
Русские Хиты 80-90-Х – Ирина Алегрова – Привет Андрей скачать бесплатно песню и слушать онлайн в mp3
Русские Хиты 80-90-Х – Ирина Алегрова – Привет Андрей
Rasa – Под Фонарем скачать бесплатно песню в mp3
Rasa – Под Фонарем
Скачать песню Каспийский Груз – Guantanamera (Izzamuzzic Remix) бесплатно в mp3
Каспийский Груз – Guantanamera (Izzamuzzic Remix)
Stargazers Fea Katty Heath – Be Here With Me (Extended Mix) скачать песню в mp3 и слушать онлайн бесплатно
Stargazers Fea Katty Heath – Be Here With Me (Extended Mix)
Украинские Хиты – Марина І Компанія – Весна скачать песню бесплатно на телефон и слушать онлайн в mp3
Украинские Хиты – Марина І Компанія – Весна
Владимир Высоцкий – Об Аэрофлоте I. Москва – Одесса (Мне Туда Не Надо) (Об Аэропортах) скачать в mp3 и слушать онлайн бесплатно
Владимир Высоцкий – Об Аэрофлоте I. Москва – Одесса (Мне Туда Не Надо) (Об Аэропортах)
Ramil – Levis скачать песню бесплатно в mp3 и слушать онлайн
Ramil – Levis
Миша Королев – Цепи Сдую скачать бесплатно песню и слушать онлайн в mp3
Миша Королев – Цепи Сдую
Мияги & Эндшпиль – Ты Моя Химия скачать бесплатно песню в mp3
Мияги & Эндшпиль – Ты Моя Химия
Скачать песню Ярмак – Сердце Пацана бесплатно в mp3, текст песни, смотреть клип
Ярмак – Сердце Пацана, текст песни, смотреть клип
Klavdia Petrivna – Імператори скачать песню бесплатно на телефон и слушать онлайн в mp3
Klavdia Petrivna – Імператори
Collini _ Eminem – My Name Is (Collini Moombahleg Edit) скачать песню в mp3 и слушать онлайн бесплатно
Collini _ Eminem – My Name Is (Collini Moombahleg Edit)
Fantastic read! I was especially impressed by the depth provided on the topic, offering a perspective I hadn’t considered. Your insight adds significant value to the conversation. For future articles, it would be fascinating to explore more to dive deeper into this subject. Could you also clarify more about the topic? It caught my interest, and I’d love to understand more about it. Keep up the excellent work!